METAL MATTERS – Why rust never sleeps…
When people see metal changing colour or breaking down, they often call it rusting — but did you know only iron-based metals can rust? Let’s take a closer look at what’s really going on with different metals when they react with air, moisture, or time.

What Exactly Is Rust?
Rust is a specific type of corrosion that affects iron and steel. It’s a chemical reaction — iron + water + oxygen = iron oxide, better known as rust.
This reaction speeds up in salty or damp environments, like coastal areas. That’s why metalwork near the sea tends to rust faster.
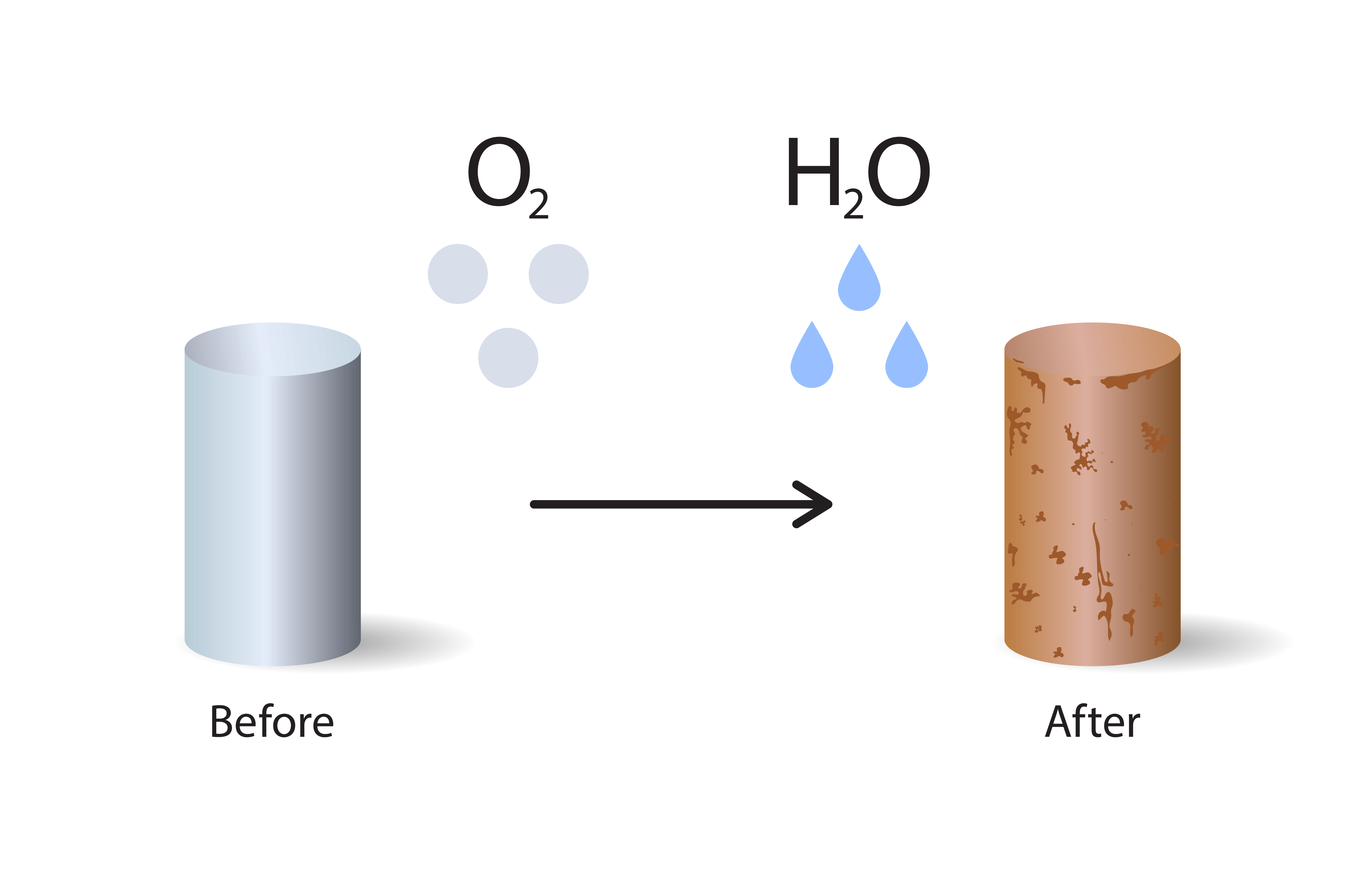
Not All Metal Corrosion Is Rust
Over time, many metals change colour, tarnish, or develop a dull surface — but that’s not the same as rusting.
A few common examples:
Copper, Bronze, and Brass – The Patina Effect
- Ever seen a statue with a blue-green finish? That’s not rust — it’s called patina.
- Copper reacts with air and moisture to form this surface layer.
- Bronze (copper + tin) and brass (copper + zinc) do the same.
- Patina is only surface-level, and doesn’t eat away at the metal like rust does.
- In fact, many people like this aged look – it’s protective and attractive!
Aluminium – Dulls, But Doesn’t Rust
Aluminium also reacts with air, forming aluminium oxide – but here’s the twist:
- The oxide layer is stable and protective.
- It actually helps prevent further corrosion.
- Unlike iron, aluminium won’t rust away over time.
Corten Steel – Rust That Protects
Corten steel, also known as weathering steel, is designed to form a stable, rust-like patina that actually protects the metal beneath.
- The surface layer seals itself against moisture and oxygen.
- The result is a warm, earthy appearance that changes over time.
- It’s popular in architecture and sculpture for its rugged, low-maintenance finish (Antony Gormley’s Angel of the North is a prime example).
Rust-Resistant Steel? Sort Of.
- Some steels are marketed as “rust-proof,” but a better term is rust-resistant.
- Stainless Steel: When chromium reacts with oxygen in the air it forms a very thin invisible film of chromium oxide on the surface, meaning oxygen and moisture can’t easily pass through it.
- Galvanised Steel: Coated with zinc, which oxidises before the steel does.
- These materials are more durable, but given enough exposure to moisture, salt, or wear, even they can begin to corrode.
Quick Fact
All rust is corrosion… but not all corrosion is rust!

Understanding the difference helps when selecting the right metal for your job — and knowing how to protect it over time.
Further reading…
Discover our A to Z guide to the Elements in Steel and learn how each element shapes the strength, quality, and performance of steel.
